 A new look into Americans’ views on health privacy from Morning Consult provides a current snapshot on citizens’ concerned embrace of technology — worried pragmatism, let’s call it.
A new look into Americans’ views on health privacy from Morning Consult provides a current snapshot on citizens’ concerned embrace of technology — worried pragmatism, let’s call it.
This ambivalence will flavor how health citizens will adopt and adapt to the growing digitization of health care, and challenge the healthcare ecosystem’s assumption that patients and caregivers will universally, uniformly engage with medical tools and apps and technologies.
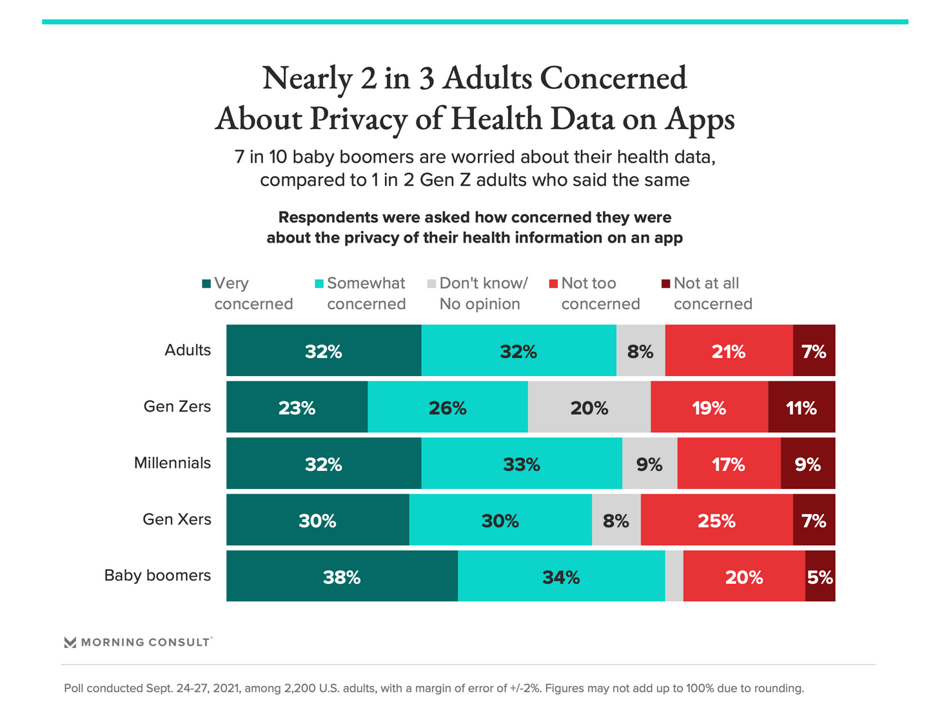
More Boomers are concerned with health data app privacy than Gen Z consumers, as the chart illustrates.
46% of U.S. adults said that health monitoring apps were not an invasion of privacy; 32% said they were, Morning Consult found.
Even so, 2 in 3 adults said they would likely get an app to track a medical condition that was approved by the FDA.
At the same time, 2 in 3 people were also concerned aobut the privacy of their health information on apps.
 And there’s the ambivalence of “concerned embrace” of digital health. The phrase “concerned embrace” was coined in a 2017 Deloitte consumer study on mobile technology trends.
And there’s the ambivalence of “concerned embrace” of digital health. The phrase “concerned embrace” was coined in a 2017 Deloitte consumer study on mobile technology trends.
That ethos now pertains to post-pandemic health consumers’ growing use of wearable tech, remote health monitoring, and mobile apps for self-care and chronic condition management.
Morning Consult polled 2,200 U.S. adults in late September 2021, a year and a half into the COVID-19 pandemic.
That timing is important because the coronavirus re-shaped how people perceived public and individual health, and the growing opportunity to adopt digital health tools for contactless care and care at-home and closer-to-home.
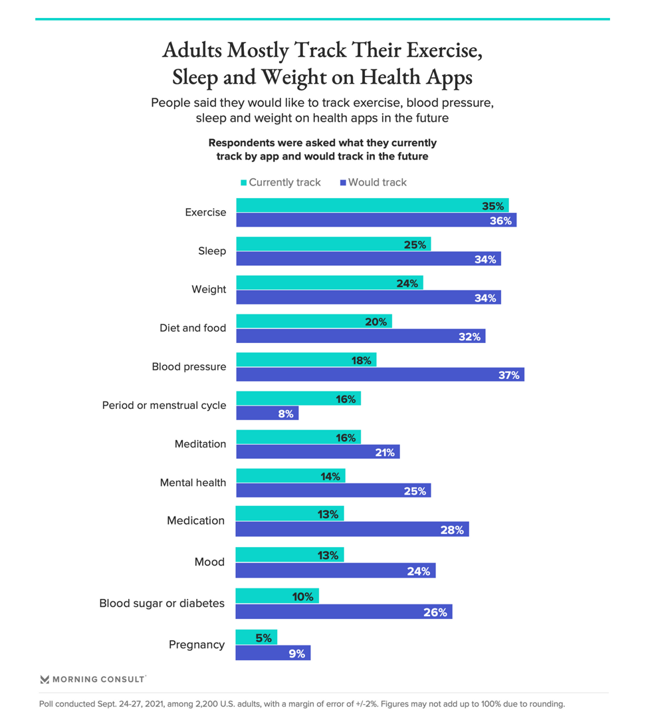
The most popular ways people currently track their health is for exercise, sleep, weight, diet and food, and blood pressure.
Looking to what people would like to track, blood pressure is first most in demand (for 37% of U.S. consumers), followed by exercise, sleep, weight, nutrition, and then new things like medication, mental health, mood, meditation, and blood sugar all attracting at least 1 in 5 new health tracking people.
The biggest deltas in terms of growing interesting are seen in this survey for:
- Blood pressure, doubling in interest from 18% current tracking to 37% looking to track in the future
- Blood sugar or diabetes, by 2.5 times (10% to 26%)
- Mood nearly doubling in interest to 24%
- Medication management more than doubling to 28%, and
- Mental health nearly doubling to 25%.
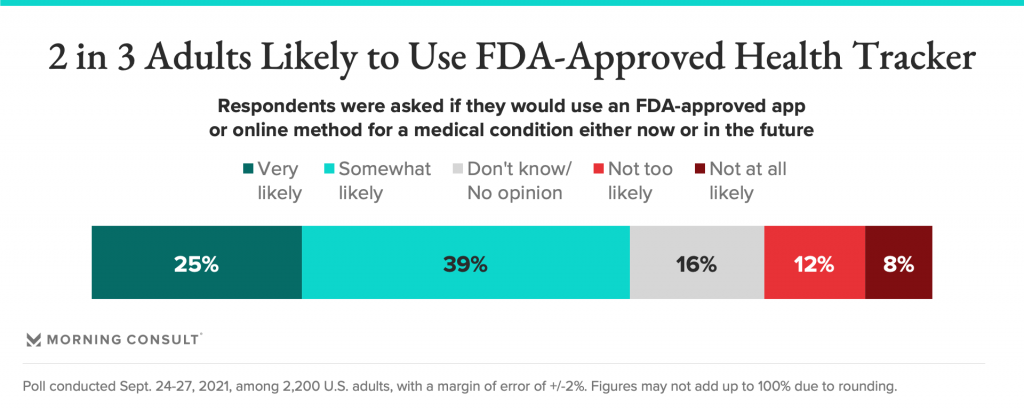 FDA clearance makes a difference in peoples’ trust with health apps, with 2 in 3 U.S. consumers likely to use an FDA-approved health tracking tool. Only 20% of people said they wouldn’t be likely to use such a digital health device.
FDA clearance makes a difference in peoples’ trust with health apps, with 2 in 3 U.S. consumers likely to use an FDA-approved health tracking tool. Only 20% of people said they wouldn’t be likely to use such a digital health device.
Other research recently conducted by Morning Consult looks into peoples’ trust in institutions, such as government, financial services, media, and indeed, with technology.
For many years, tech held high trust-equity with consumers, attracted to the convenience of mobile phones and ecommerce, and connections via social media.
Today, not so much…as we see Facebook facing greater political and regulatory scrutiny in the U.S. Google confronting similar pressures in the European Union, and growing privacy breaches facing citizens the world over. For our planning forecast and building in privacy-by-design, we should expect growing concerned-embraces of tech among the mainstream of people.
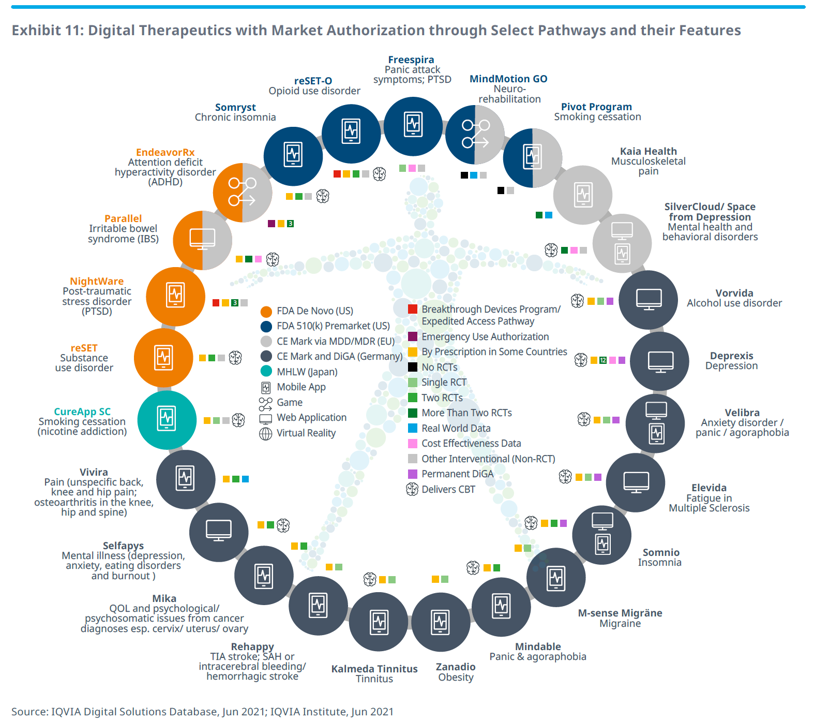 Health Populi’s Hot Points: The latest IQVIA report on digital health apps includes an in-depth analysis of regulatory pathways across countries. The digital therapeutics wheel shown here from IQVIA’s work illustrates some of the digital health tools with a lens on various national and regional regulatory regimes evaluating each of them.
Health Populi’s Hot Points: The latest IQVIA report on digital health apps includes an in-depth analysis of regulatory pathways across countries. The digital therapeutics wheel shown here from IQVIA’s work illustrates some of the digital health tools with a lens on various national and regional regulatory regimes evaluating each of them.
These efforts, each assessing evidence bases and real-world information, will help to bolster health citizens’ confident adoption in the tools when they are cleared or approved by the agencies.
In the wake of COVID-19, one key learning of people living through the public health crisis is gaining a greater understanding of and appreciation for the work of the FDA and other regulators who aim to ensure public safety and health produce efficacy. [This isn’t a universal belief among all health citizens, but a majority still embracing science for medicine].
Rational and evidence-based regulation for digital health tools that bake software into self-care, coupled with peoples’ growing health engagement, are setting the stage for digital health adoption in peoples’ hands as homes continue to morph into personal and family health hubs and new home care life-flows.
Adopting privacy-by-design as a rule — through the eyes and values of end-users — should be front-of-mind among all digital health innovators.
